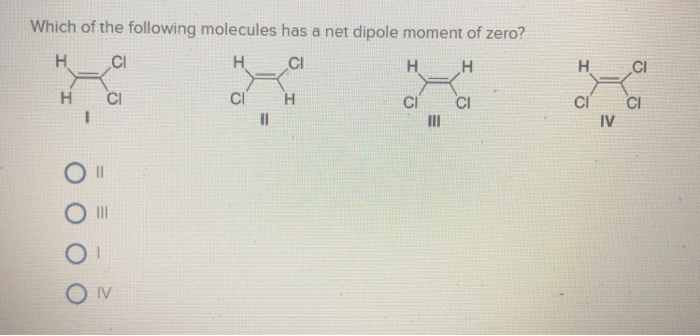
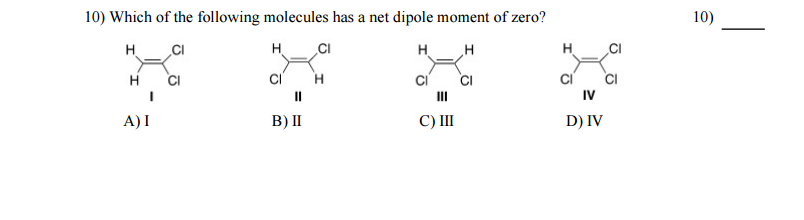
Which of the Following Molecules Has a Net Dipole Moment
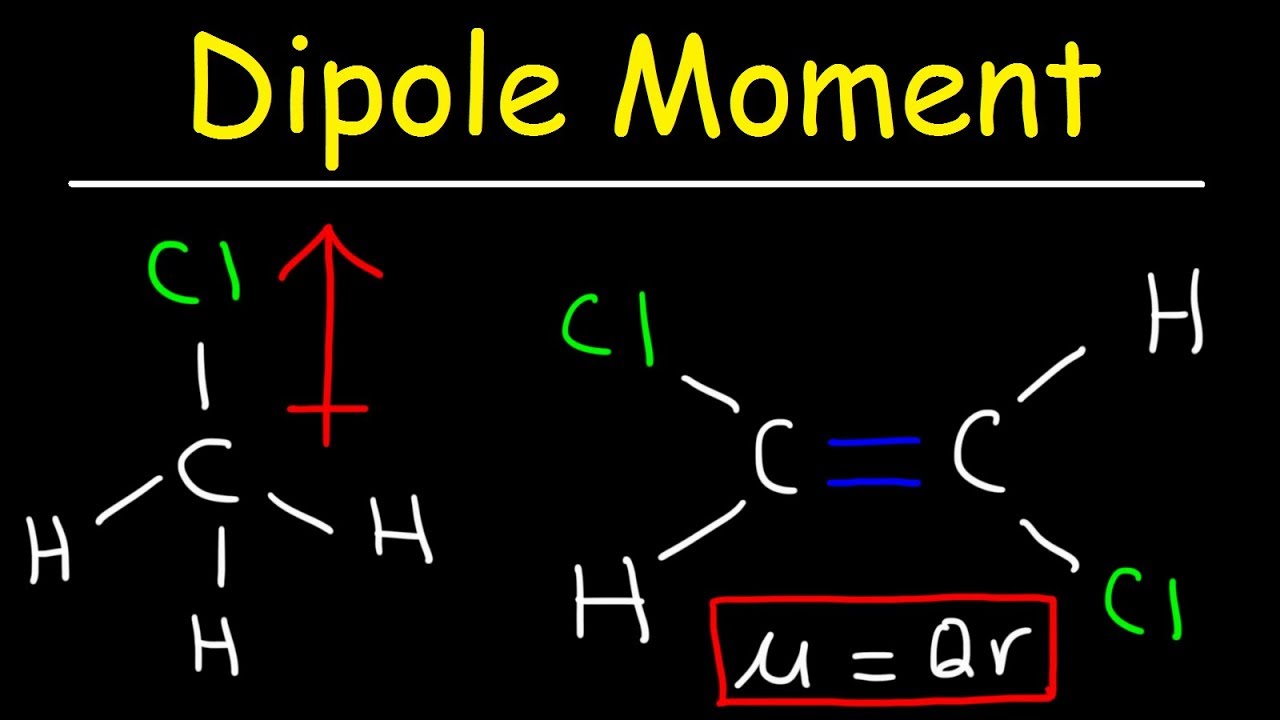
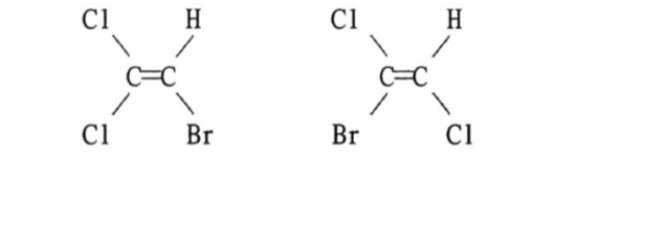
The dipole moments of the C-Cl bonds in cis-12-Dichloroethene reinforce and the molecule exhibits a net dipole. This occurs due to an atoms electronegativity - where one atom has the ability to attract electrons towards it In other words electrons wants to spend other time around it giving it a negative charge and the other a positive charge.
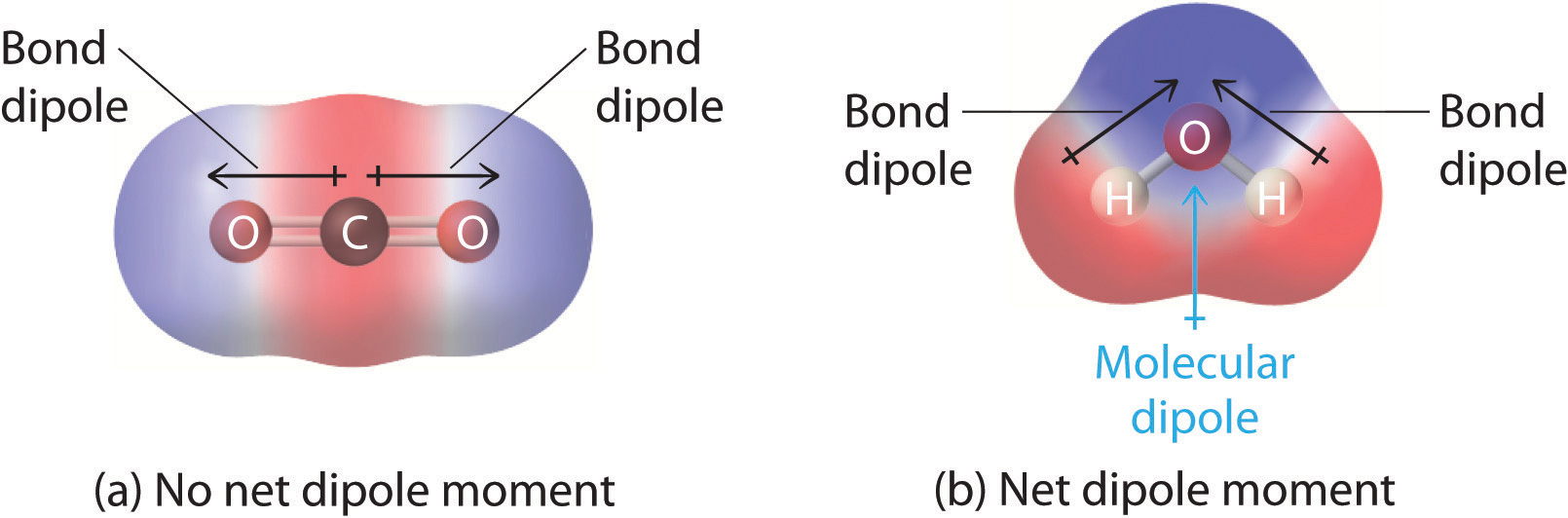
Carbon dioxide has zero dipole moment but the structure is not tetrahedral.

. Which of the following molecules has a net dipole moment of zero. Moment is not zero. Who are the experts.
They are all nonpolar. Dipole moment if there is difference in electronegativity between two atoms. Dipole arrows to show bond polarity for each bond in the following molecules.
A I B II C III D IV. Dipole moment is a vector quantity ie. Whereas other molecules such as C H 4 C O 2 and C C l 4 have zero dipole moment as the bond dipoles completely cancel each other.
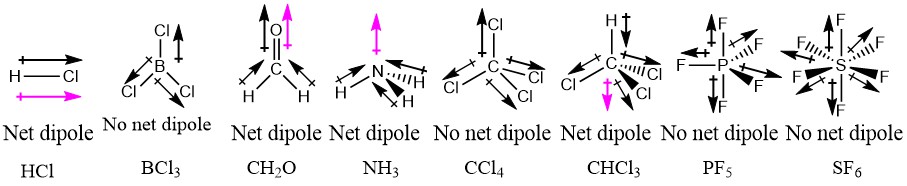
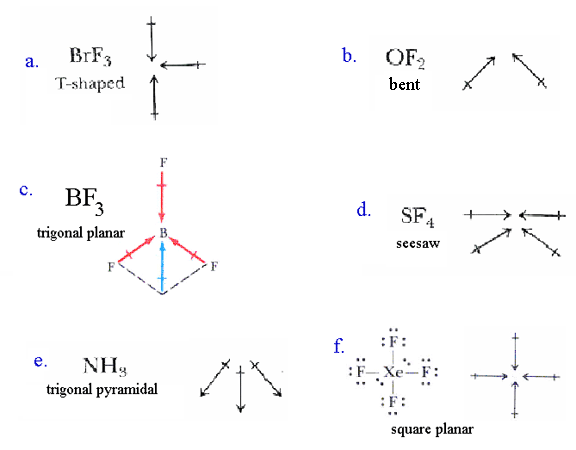
Net dipole operates on the same idea - but it focuses on the direction and. Which of the following possess net dipole moment. Due to their different three-dimensional structures some molecules with polar bonds have a net dipole moment HCl CH2O NH3 and CHCl3 indicated in blue whereas others do not because the bond dipole moments cancel BCl3 CCl4 PF5 and SF6.
It has a permanent dipole moment. Then state whether. In contrast the trans isomer has no molecular dipole since the C-Cl bonds are at the opposite direction and their dipole effects are canceled.
According to VSEPR theory a molecule with the general formula AX 6 will have a _____ molecular shape. Question 2 Which of the following molecules has a net dipole moment. All molecules with polar bonds have a permanent dipole moment.
The molecule will have a net dipole moment ie whether each molecule is pol ar or nonpolar. SF 2 Correct Answer. C H C l 3 has net dipole moment as the bond dipoles do not cancel each other.
Carbon tetrachloride and tin tetrachloride have tetrahedral structure and zero dipole moment because all the bond dipoles cancel each other which gives net dipole moment zero. A BeCl2 B SF2 C KrF2 D CO2 E CCl4. Which of the following molecules does not have net dipole moment.
CO2 CCl4 NF3 CS2 COS F2 SO3 H20 x Check all of the molecules which have a net dipole moment. 1 Approved Answer. 5 Ratings 18 Votes The correct option is A ie.
Select all of the following that would have a net dipole moment. This problem has been solved. Check Answer and Solution for above question from Chem.
Only molecules with polar bonds may have a permanent dipole moment. 53 Which of the following molecules have net dipole moments. For the molecules that are pola1 indicate the polarity of each bond and the direction of the net dipole moment of the molecule.
A CCl4 B BF3 C CO2 D NH3. Which molecules have a net dipole of zero. The polar bonds in the bent H2O molecule result in a net dipole moment so H2O is polar.
All square planar molecules are nonpolar. Non-polar Net dipole to the lone pair Nitrogen is slightly more electronegative than Br. It has a bent structure due to the presence of one lone pair.
Linear molecules cannot have a net dipole moment. Carbon tetrachloride and tin tetrachloride have tetrahedral structure and zero dipole moment because all the bond dipoles cancel each other which gives net dipole moment zero. Check Answer and Solution f.
Experts are tested by Chegg as specialists in their subject area. A BeCl2 B CO2 C SO2 D BF3. See the answer See the answer done loading.
Out of the following which compound has zero dipole moment. Science Chemistry QA Library Which of the following molecules have net dipole moments. Which of the following molecules has a dipole moment.
Due to their different three-dimensional structures some molecules with polar bonds have a net dipole moment HCl CH2O NH3 and CHCl3 indicated in blue whereas others do not because the bond dipole moments cancel BCl3 CCl4 PF5 and SF6. A CH3Br B CH2Cl C HCOOH D Image D. What are the 4 principle buffers in.
Is a polar molecule. In it the individual H-S bond moments do not cancel out each other so that the net dipole. It has magnitude as well as direction.
Do all polar molecules have a net dipole moment. BCl 3 NBr 3. ClNO N is the central atom.
Polar molecules have a non-zero net dipole moment. It has a permanent dipole moment. Both CO2 and H2O have two polar bonds.
They can have a net. Dipole moments occur when there is a separation of charge. Which of the following molecules has a net dipole moment which one is polar.
Which of the following molecules does not have a net dipole moment of zero. Individual bond dipole moments are indicated in red. In HCl the molecular dipole moment is equal to the dipole moment of H-Cl bond ie 107 D.
Between all these molecules NH3 will have net dipole moment as it is pyramidal in shape with 1 lone pair of e- on the top of the pyramidand 3-H atoms going downThe bond dipoles of three N-H in this molecule do not cancel each other thus net dipole moment is of 149DBesides this the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of 3 N-H. PCl3 P is the central atom D. SF 2 Correct Answer.
Shilpy J answered on January 26 2021. However the dipoles in the linear CO2 molecule cancel each other out meaning that the CO2 molecule is non-polar. Asked Jun 23 2017 in Chemistry by Jamie.
Group of answer choices CH4 CCl4 SO2 None. C S 2 is one of the molecules which does not possess a dipole moment. A CH3Cl b CHCl3 c CCl4 d CHI3 asked Apr 10 2020 in Halogen Derivatives by Rukmani 511k points.
Moment is not zero. SF 2 Question 3. As a polar diatomic molecule possesses only one polar bond the dipole moment of that molecule is equal to the dipole moment of the polar Bond.

Solved Consider The Following Molecules Which Of These Chegg Com
Dipole Moment Molecular Polarity Percent Ionic Character Youtube

4 Pts Based On The Molecular Structure And Net Dipole Moment Predict Whether Each Of The Homeworklib
1 4 Polar Covalent Bonds Dipole Moments Chemistry Libretexts

Molecular Dipole The Overall Polarity Of The Molecule Chemistry Steps
.png?revision=1)
2 2 Polar Covalent Bonds Dipole Moments Chemistry Libretexts
Solved Which Of The Following Molecules Has A Net Dipole Chegg Com

Molecular Dipole The Overall Polarity Of The Molecule Chemistry Steps
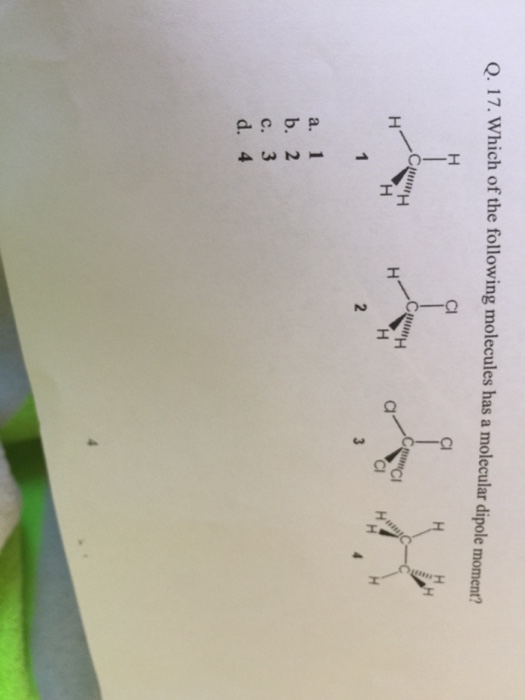
Solved Which Of The Following Molecules Has A Molecular Chegg Com
Solved Which Of The Following Molecules Have A Net Dipole Chegg Com
How To Predict Whether A Molecule Has A Dipole Moment
Solved Which Of The Following Molecules Has A Net Molecular Chegg Com
2 2 Polar Covalent Bonds Dipole Moments Chemistry Libretexts

Dipole Moments Mcc Organic Chemistry

How To Determine Whether A Molecule Has An Overall Molecular Dipole Moment Youtube
Solved Which Of The Following Molecules Has A Net Dipole Chegg Com

Which Of The Following Molecule Has A Net Dipole Moment Youtube

Molecular Dipole The Overall Polarity Of The Molecule Chemistry Steps

Which Of The Following Molecules Does Not Have Net Dipole Moment Youtube

Comments
Post a Comment